Discover the capabilities of our AI-powered GxP audit in a live session. We’d be happy to demonstrate the tool’s features—using your real data, if desired.
Dr. Peter Göbel
Associate Partner
+49 176 13546017
Peter.Goebel@consileon.de
Consileon supports pharmaceutical manufacturers in efficiently reviewing processes, supply chains, and documentation for compliance with Good Distribution Practice (GDP) guidelines – automated, traceable, and language-independent.
Pharmaceutical companies are subject to strict regulatory requirements under the Good Distribution Practice (GDP) guidelines, which safeguard the quality, integrity, and traceability of medicines throughout the entire supply chain—from international suppliers to in-house storage, all the way to delivery to pharmacies, hospitals, or wholesalers.
To demonstrate GDP compliance, pharmaceutical companies must regularly undergo comprehensive audits. These reviews are time-consuming, complex, and demand a high degree of precision. Adding to the challenge, rising regulatory requirements meet a limited pool of experienced auditors—driving up costs. Non-compliance with GDP can have serious consequences, ranging from license revocation and heavy fines to reputational damage and legal risks.
Consileon offers an AI-powered solution that helps pharmaceutical companies streamline the review of GxP-relevant processes, policies, and documents, and prepare for certification. To address the global nature of pharmaceutical supply chains, the AI solution is designed for multilingual use.

Pharmaceutical manufacturers must demonstrate GDP compliance comprehensively, transparently, and efficiently. Consileon combines deep industry expertise with modern technology to enable a time-saving and reliable review process.
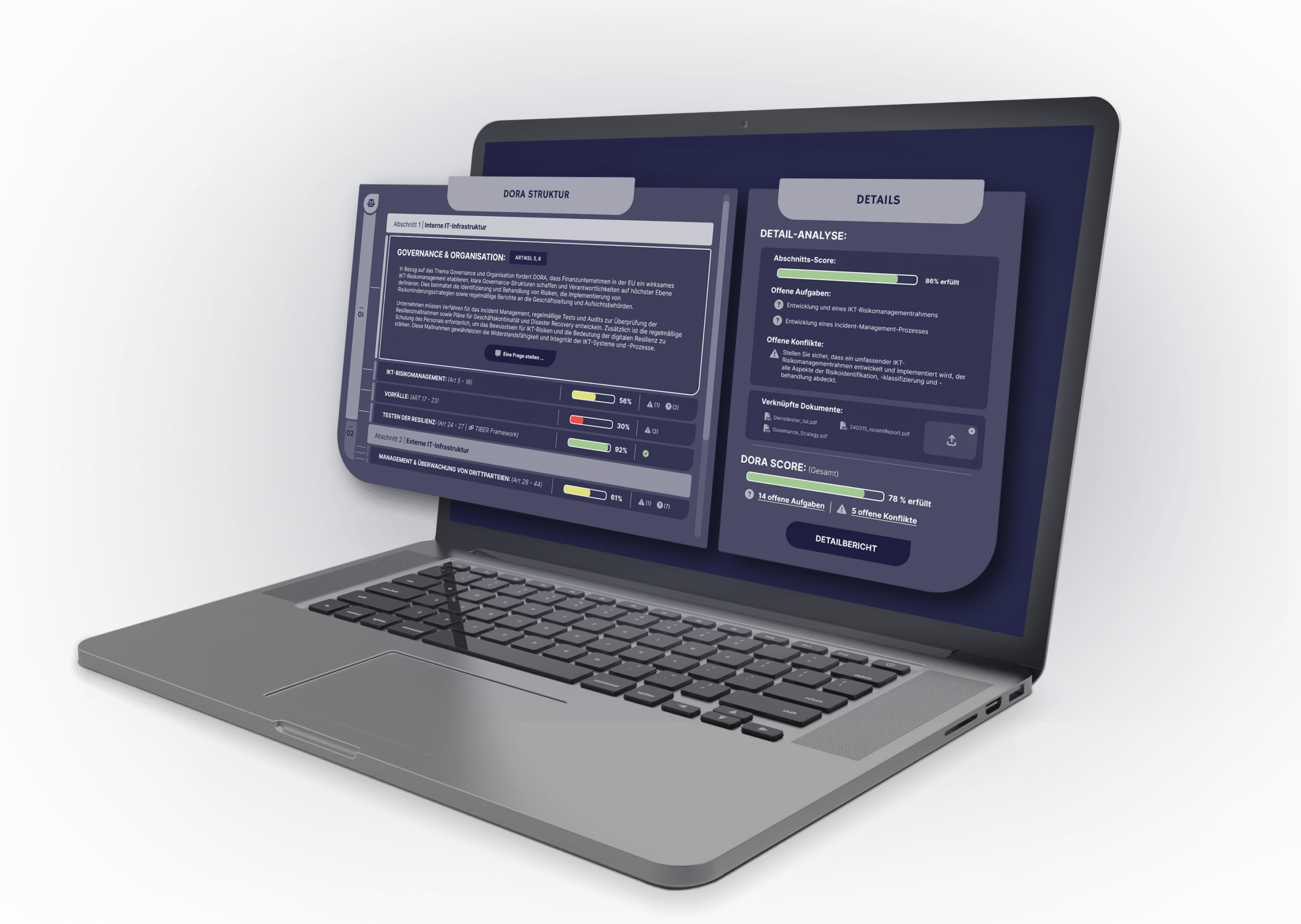
Our software is powered by a unique body of knowledge derived from numerous GDP audits. This has led to the development of a catalog containing over 300 main questions and hundreds of sub-questions, forming the foundation of every GDP audit. Previously, all required documents—such as internal quality management policies, supplier records, or delivery notes from domestic and international sources—had to be manually reviewed and matched against these questions. We have transferred this comprehensive audit expertise into a powerful Large Language Model (LLM) that automatically analyzes and assesses documents, regardless of format or language, and provides a clear status on GDP compliance. The results are quality-assured: Our AI models are continuously monitored by Lighthouz AI to ensure full compliance with the EU AI Act.
Once set up, GDP auditors can easily upload relevant documents through an intuitive interface or API. Naturally, your data is securely protected at all times. Thanks to multi-tenant architecture, no one else can access your SOPs, audit protocols, or other sensitive documents. The AI automatically analyzes content—regardless of language—to assess GDP compliance. It uncovers critical gaps, potential errors, or ambiguous language using a comprehensive set of audit questions. The results are presented in a familiar traffic light system: Green indicates full compliance, Yellow highlights minor issues or ambiguities, Red signals urgent action is required.
Despite automation, human expertise remains crucial to the process. For every assessment, the tool provides a detailed explanation along with the exact text reference from the reviewed document. This allows the auditor to transparently understand the AI’s reasoning and independently determine whether to confirm or override its assessment—in line with the Human-in-the-Loop principle. As a result, the final evaluation remains in expert hands, ensuring the highest standards of quality and accountability.
The next step is the on-site verification. The auditor ensures that documented processes and conditions are actually implemented in practice—a critical element of every audit. By handling the time-consuming document analysis, AI frees up auditors to focus more thoroughly on this essential component, reducing the burden of sifting through (digital) paperwork.
Finally, the system generates comprehensive reporting on GDP compliance. This report can be used internally for documentation purposes and externally as proof for authorities or certification bodies—in a manner that is transparent, verifiable, and audit-proof.
Our AI solution ensures maximum security for your sensitive data. No permanent storage or training of the AI with your documents takes place. All data is processed exclusively in encrypted form and remains within the EU, fully compliant with the GDPR. Through strict access and segregation management, it is guaranteed at all times that unauthorized parties cannot gain access. In addition, mechanisms such as DDoS protection safeguard the availability of the application. The development follows the proven standards of OWASP, ensuring that your data remains secure and reliably protected at all times.

The GDP audit is just one of many regulatory challenges faced by pharmaceutical companies. At Consileon, you’ll be supported by experts with deep knowledge of pharmaceutical regulations. Our AI-powered solutions are flexible and scalable, supporting not only GDP audits but also helping you comply with other GxP standards, such as GMP (Good Manufacturing Practice) and GLP (Good Laboratory Practice). This enables you to ensure consistent quality, minimize regulatory risks, and maintain compliance throughout the entire product lifecycle—from manufacturing and storage to distribution.
With Consileon’s AI-enabled audit solution, pharmaceutical companies benefit from measurable advantages:
Reduce audit effort through automated document and process review

Enhance audit reliability with quality-assured AI analysis
Faster results with clear actionable recommendations using a traffic light system
Multilingual: review international supplier documents without the need for translation

Flexibly extendable to other GxP requirements such as GMP or GLP
Audit-proof documentation for internal and external requirements
Data security ensured through multi-tenant architecture, GDPR-compliant data protection guaranteed

Efficient CAPA assessment of Corrective and Preventive Actions

Consistent quality through machine-based review, even during lengthy and large-scale audits
The GDP audit solution is built on the Consileon Regulatory Radar, pre-configured for GDP audits and available as a SaaS application. It integrates seamlessly into existing audit and quality assurance workflows, delivering fast, transparent results, reduced audit workload, and secure GDP certifications. Once implemented, Consileon offers an attractive pay-per-use model.
Discover the capabilities of our AI-powered GxP audit in a live session. We’d be happy to demonstrate the tool’s features—using your real data, if desired.
Dr. Peter Göbel
Associate Partner
+49 176 13546017
Peter.Goebel@consileon.de
"*" indicates required fields